Abstract

Background: Children and adolescents/young adults (AYAs) with NCI high-risk Philadelphia chromosome-like acute lymphoblastic leukemia (Ph-like ALL) experience high relapse rates and poor clinical outcomes with conventional chemotherapy. Approximately 70% of Ph-like ALL is driven by oncogenic kinase fusions or mutations that induce constitutive JAK/STAT signaling, including CRLF2 rearrangements with frequent JAK2 co-mutations, JAK2 rearrangements, EPOR rearrangements, SH2B3 deletions, and IL7R indels. The Part 2/efficacy phase of the Children's Oncology Group (COG) AALL1521/INCB18424-269 phase 2 trial (NCT02723994) is testing the hypothesis that addition of the selective JAK1/2 inhibitor ruxolitinib to the standard of care high-risk B-ALL chemotherapy backbone will improve 3-year event-free survival (EFS) of children and AYAs with the most common CRLF2-rearranged (R) subtype of Ph-like ALL (n=84 patients; Cohorts A and B). After exploration of 6 dose levels, the recommended phase 2 dose of ruxolitinib in combination with chemotherapy was identified as 50 mg/m2/dose x 14 days on/14 days off per cycle in the Part 1/safety phase of AALL1521 conducted in 40 pediatric patients (Tasian ASH 2018 #555). We now report the biologic characteristics of patients with non-CRLF2-rearranged Ph-like ALL enrolled in the descriptive Cohort C of AALL1521 and their early treatment responses by minimal residual disease (MRD) assessment.
Methods: Children and AYAs aged 1-21 years with Ph-like ALL and eligible JAK pathway genetic alterations in Cohort C were enrolled in Part 1 (September 2016 to August 2018) or Part 2 (August 2018 to August 2019) for treatment with ruxolitinib and post-induction chemotherapy. Genetic characterization of diagnostic bone marrow or peripheral blood ALL specimens was performed via 8-gene low-density microarray (LDA) profiling for the Ph-like gene expression signature, fluorescence in situ hybridization, RNA-based kinase fusion analysis, and/or DNA-based next generation sequencing in COG reference laboratories as described (Harvey & Tasian Blood Adv 2020). End of induction (EOI) and end of consolidation (EOC) MRD assessment was performed via flow cytometry analysis with positive MRD defined at 0.01%.
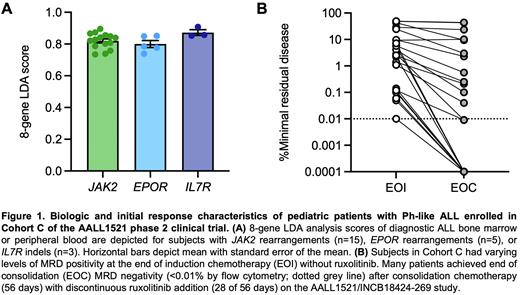
Results: Within Cohort C, 5 patients with non-CRLF2-R Ph-like ALL were enrolled in Part 1 and 18 patients in Part 2. The mean age and diagnostic white blood cell counts were 13.3 years and 145,700/μL blood, respectively. Fifteen patients had JAK2 fusions (with 3’ partners EBF1, HAUS6, HOOK3, PAX5, SNX29, SSBP2, or TERF2), 5 had EPOR rearrangements (with 3’ partners IGH or MTRNR2L8), and 3 had IL7R indels. Ph-like LDA scores were uniformly high in Cohort C cases with a mean value of 0.826 (Figure 1A). High EPOR expression level (DCt <4) on LDA was predictive of true EPOR rearrangement identified on fusion analysis. All Cohort C patients had residual disease after 4-drug induction chemotherapy (without ruxolitinib) with mean EOI MRD 10.7% (range 0.01-49%). Two of 5 patients (40%) in Parts 1 and 8/18 patients (44.4%) in Part 2 achieved EOC MRD negativity (Figure 1B). Two Part 1 and 9 Part 2 patients (including two patients with EOC MRD+ at 0.04% or 0.1%) continued post-consolidation chemotherapy with ruxolitinib with several patients now status post therapy completion. One additional Part 2 Cohort C patient with a JAK2 fusion and negative EOC MRD proceeded to elective hematopoietic stem cell transplantation in first remission outside of the AALL1521 trial.
Conclusions: Patients with Ph-like ALL harboring JAK2 or EPOR rearrangements or IL7R indels routinely had higher LDA scores and levels of EOI MRD positivity versus those with the more common CRLF2-R subtype (Tasian ASH 2020 #1095). JAK2 rearrangements occurred most frequently amongst Cohort C patients, often with previously-unknown gene partners. Although 13/23 (56.5%) of Cohort C patients were EOC MRD+, most patients demonstrated a marked decrement in EOI to EOC MRD with ruxolitinib and consolidation chemotherapy. The optimal duration of JAK inhibitor therapy and potential response rates in patients with Ph-like ALL remain undefined. Future analyses of MRD levels and 3-year EFS in patients with CRLF2-R +/- JAK-mutant Ph-like ALL enrolled in Cohorts A and B of the AALL1521 trial will help to ascertain potential outcomes benefit of ruxolitinib addition to chemotherapy, although responses may differ from those of Cohort C patients.
Disclosures
Tasian:GSK: Consultancy; Aleta Biotherapeutics: Consultancy; Beam Therapeutics: Research Funding; bluebird bio: Consultancy; Syndax Pharmaceuticals: Consultancy; Kura Oncology: Consultancy, Research Funding; Incyte Corporation: Research Funding. Hunter:Incyte Corporation: Current Employment. Daniel:Incyte Corporation: Current Employment, Current equity holder in private company. Raetz:Pfizer: Research Funding; BMS: Other: Data and Safety Monitoring Board. Hunger:Amgen: Current equity holder in private company, Honoraria; Jazz: Honoraria; Servier: Honoraria. Assad:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company.
OffLabel Disclosure:
This clinical trial is investigating the therapeutic potential of the JAK inhibitor ruxolitinib in combination with multi-agent chemotherapy for children, adolescents, and young adults with Ph-like ALL.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal